Ever wondered why water doesn’t dance to a rolling boil in the oven? Let’s break down the science behind this question to find out why.
You might have noticed that when you place a water pan in the oven to keep your loaf or roast moist during a long cook, the water doesn’t seem to bubble.
Is it boiling? Shoot, is it even evaporating? Or could there be something wrong with your oven? Welcome, and read on! We’ve put together this guide to help you skip the physics lesson (well, as much as possible) and get straight to the answers.
What Makes Water Boil With Bubbles?
Fill a pot with water, place it on the stovetop, turn the burner up to high, and stand by.
As the water reaches its boiling point (212°F / 100°C), you will soon witness a familiar sight: bubbles form at the bottom of the pot, rise gently to the top at first, then more vigorously, until they begin rolling and bursting uncontrollably.
The reason for this lies in two factors: First, the heat source is coming from below—the stovetop’s burner. Second, this induces the formation of convection currents in the water. Let’s break this down even further to understand exactly how it works.
When you’re heating water on the stovetop, the burner heats the bottom of the pot. The metal then transfers energy in the form of heat to the water.
The water at the bottom absorbs this energy and becomes so energized, it turns to water vapor. Hotter and no longer as dense as the liquid around it, this vapor has no option but to rise up.
Meanwhile, convection currents have formed in the water. The water molecules hot enough to move but not to transition into a gaseous state rise to the top, making room for the cooler water at the bottom to take their place.
This cooler water then heats up and rises, only to be replaced again by cooler water descending from above. This cycle continues on, and on, and on again. The convection currents help distribute the heat evenly.
Here’s what this looks like:
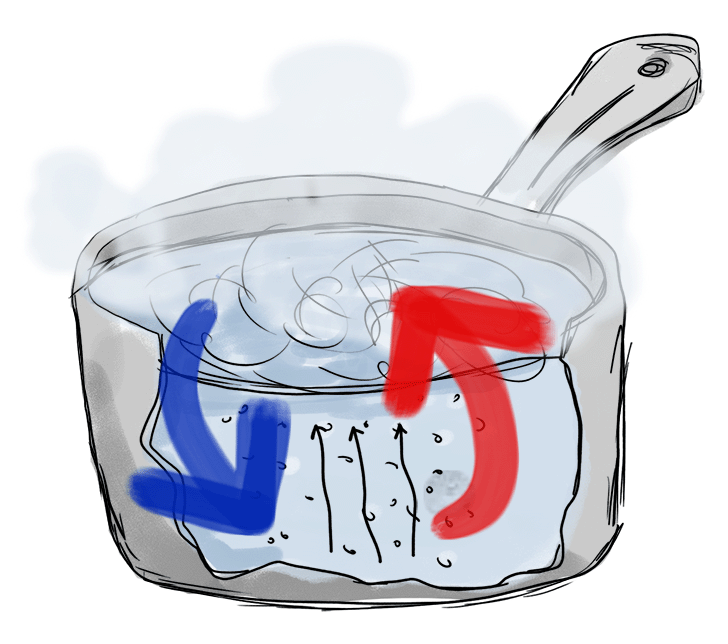
Notice the blue and red arrows representing the convection currents, the black arrows representing the water vapor, and the steam rising up from the top.
So why doesn’t water boil as vigorously in the oven?
Can Water Boil in the Oven?
Water can evaporate and even boil in the oven. It just doesn’t do so in the way most of us expect it to—with vigorous bubbles.
On the stovetop, you’re heating water rapidly and from the bottom, in a tall, narrow pot. In the oven, the heating is more gradual. It takes place from all sides and involves shallow, wide bakeware like a foil pan or a metal baking sheet.
The wide bakeware gives the water a larger surface area for evaporation. Similar to the way our bodies sweat to cool down, evaporation cools the top part of the body of water by using up energy to transition molecules from a liquid to a gas state.
Depending on the temperature in your oven, the water may be near its boiling point. The water is doing its job, turning to steam and adding humidity that keeps your loaf, roast, or turkey moist. It’s just doing so at a slower rate than on the stovetop.
If you keep the water in a hot enough oven for a long enough time, it will eventually boil. Rougher, more porous bakeware like a cast iron pan will cause bubbling; smoother, non-porous bakeware like a glass baking dish will discourage bubbling and promote superheating.
If you want to get scientific about this, you can verify it yourself:
Place a pan of water in the oven, turn up the heat, and give it time. Then point an infrared thermometer at the water to measure its surface temperature; it will be near 212°F / 100°C.
What All This Means for You
The rolling boil is mostly a stovetop phenomenon. It takes place due to a combination of several factors, including the method of heat transfer, the amount of energy involved, and the shape and form of the cookware used.
We cook foods like pasta in a rolling boil because the pot regains heat quickly after they are added to the water and the convection currents prevent the food items from sticking to the pot or one another.
We don’t need a rolling boil—or even bubbling water—in the oven, because we use the water for a different purpose.
In stovetop boiling, the water is a medium for heat transfer, like cooking in oil. However, when we add a water pan to the oven, it we do so to add humidity and steam, which help keep meats and vegetables tender, even with extended cooking periods.
Exactly how much water you need comes down to the temperature, cooking time, and bakeware used. As a general rule, higher heat, longer cooking times, and wider pans make water evaporate faster, requiring more water during cooking.
The Takeaways
Stovetops transfer heat to the cookware through the burners, which subsequently heat the water. This occurs quickly and from the bottom of the pan or pot.
Ovens, on the other hand, heat the air, which then gradually transfers heat to the bakeware and the water. This heat transfer occurs more gradually and evenly than on the stovetop, which is why water often boils without bubbles in the oven.
